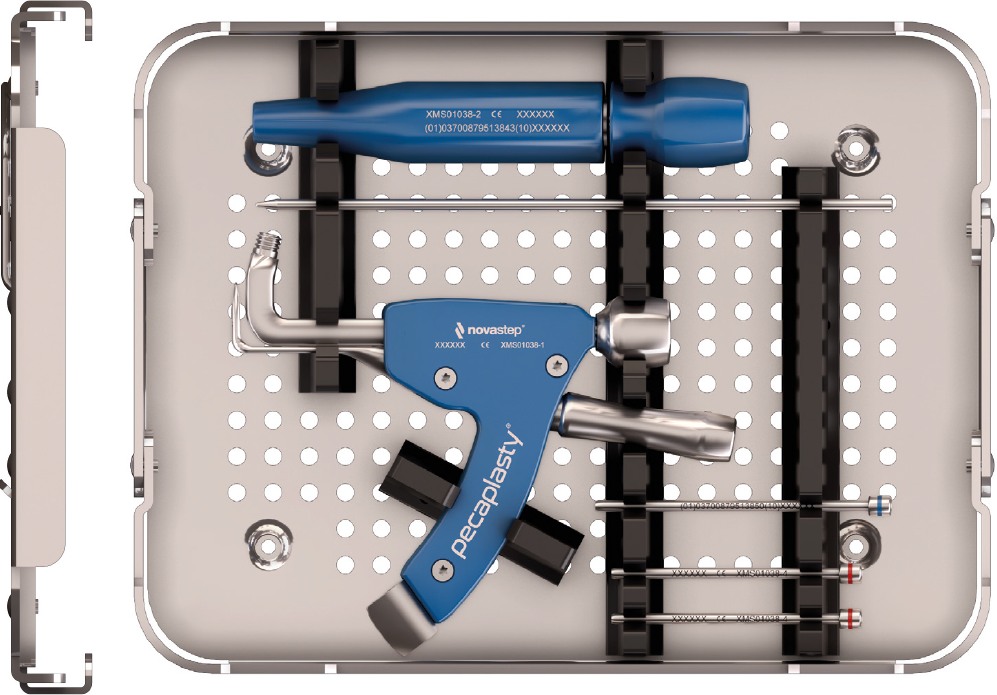
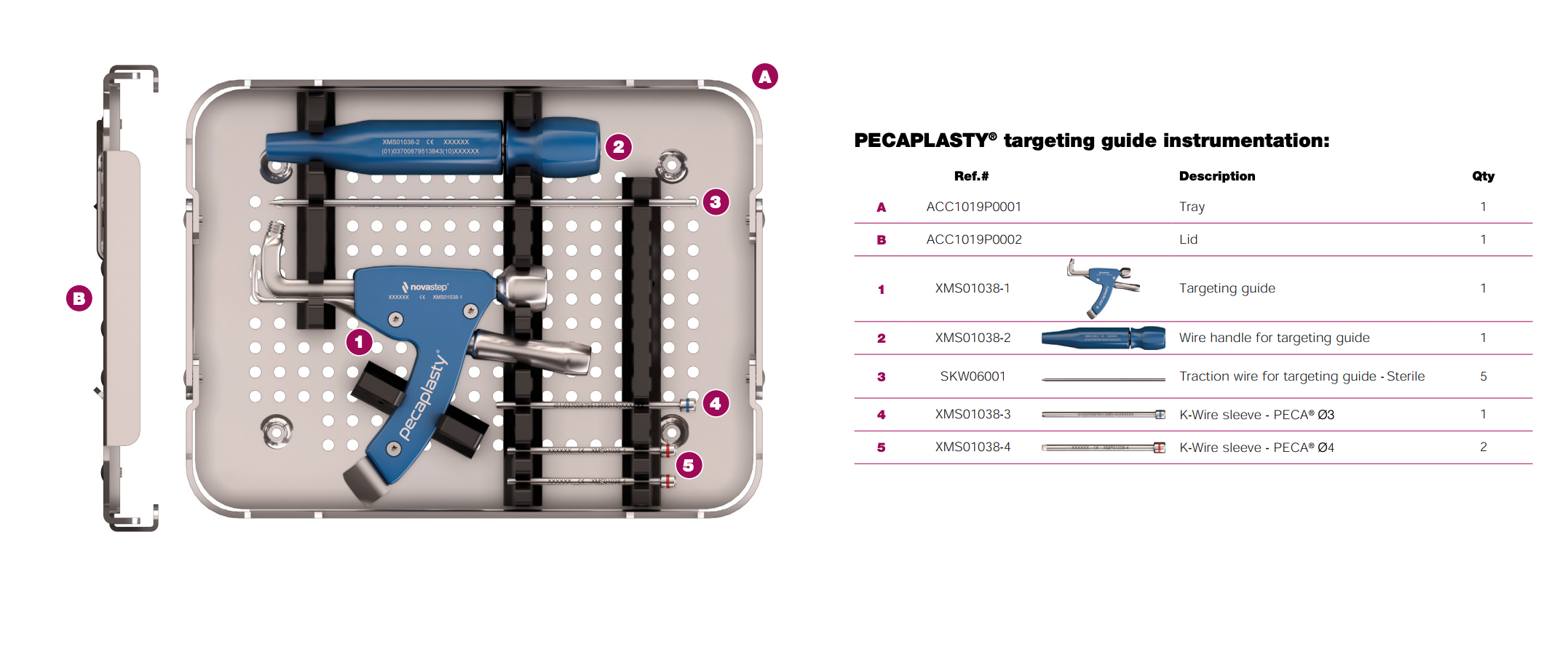
Pecaplasty® targeting guide
The Pecaplasty® System delivers reproducible outcomes in percutaneous bunion correction. The instrument helps navigate the challenges of the procedure by controlling translation and providing accurate placement of the K-wires. PECA® Implants are used to fasten and stabilize the correction during the healing process.
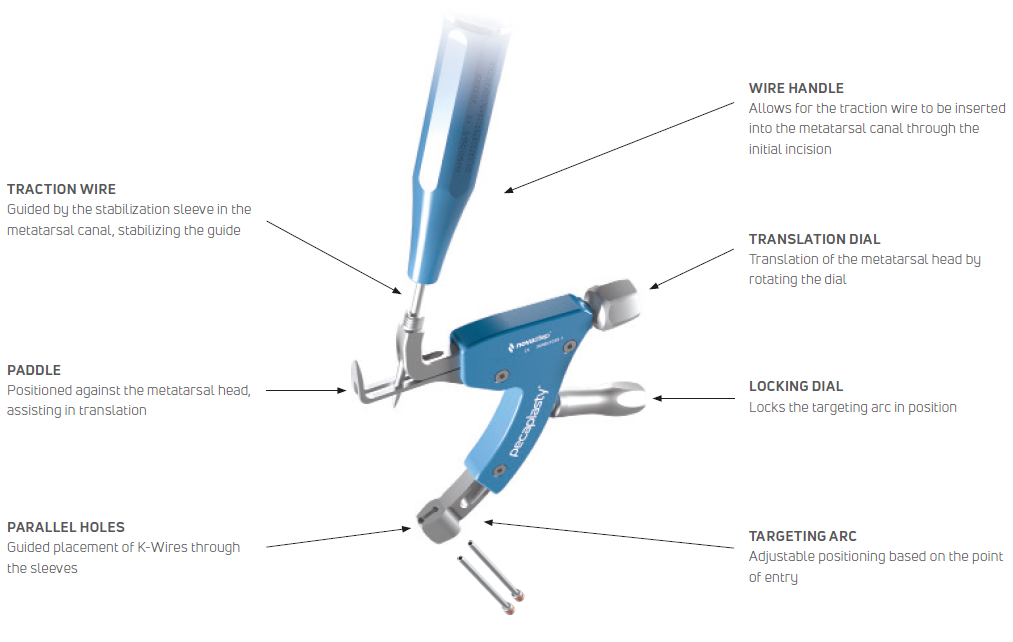
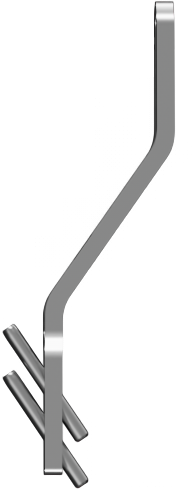
1. Easy positioning on the foot
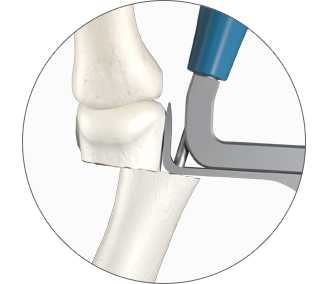
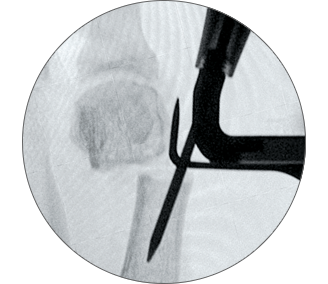
POSITION the guide by inserting the paddle underneath the medial capsule through initial incision, after a transverse osteotomy.
2. Controlled translation of metatarsal head
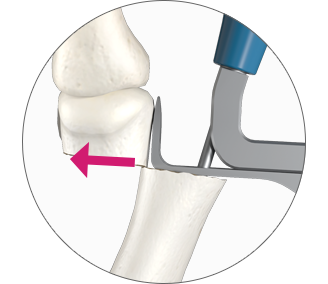
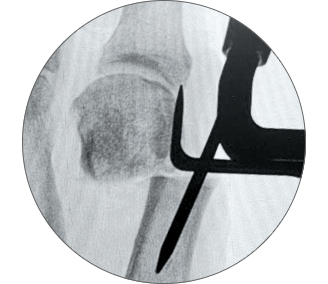
TRANSLATE the metatarsal head using the translation dial;
3. Accurate placement of K-Wires
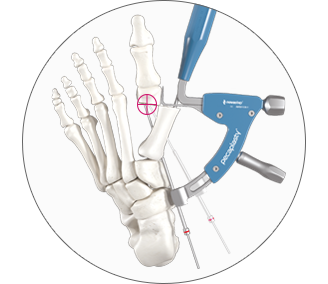
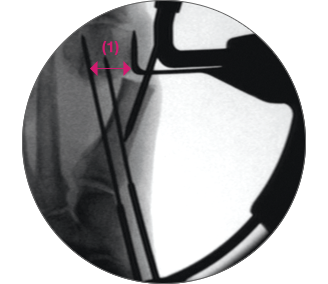
ADJUST the arc around its center of rotation to allow proper placement of the k-wires.
- Insertion point must be as proximal as possible to ensure bicortical fixation.
- Aiming point is fixed, always 14 mm (1) from the paddle.
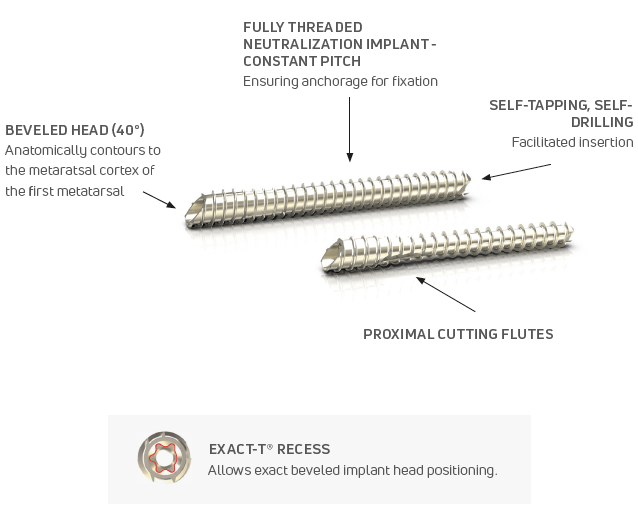
Peca® Implants
The osteosynthesis screws are indicated for arthrosis, hallux valgus and other bone alignment defaults (pes cavus, flatfoot, malalignment secondary to previous trauma).
- 40 Degree Chamfer Cut Head
- Fully Threaded, Constant Pitch
- Exact-T® 10 Recess
- Self-tapping, Self-drilling
- Proximal Cutting Flutes
Sterile Burrs

Non-contractual image
*References supplied separately
Prior to use Novastep® systems, carefully read the surgical technique, the instructions for use (IFU) and all packaging label information related to the implants and instruments.
Medical devices. Implants: Class IIb-CE1639; Instruments: Class I-CE / Class Ir-CE1639 / Class IIa-CE1639.