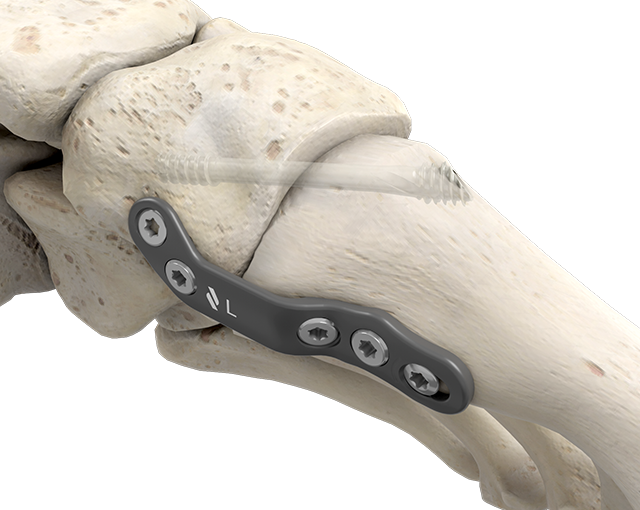
Plantar Lapidus
- Indication: first metatarsal-cuneiform fusion
- Range: Short plates / Long plates
Left & Right option - Presslock® compression hole: 3.5 mm locking screw
- Transversal screw hole: 3.5 mm locking screw
- Radius of curvature: preservation of the anterior tibial tendon

Low profile design > Plate thickness optimized according to the indications to limit subcutaneous discomfort.

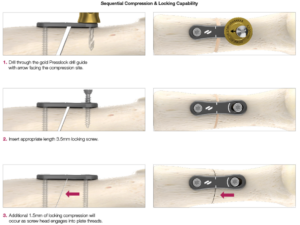
The Presslock® slot generates mechanical compression between two bone segments, before subsequent locking in the threaded part of the slot. To receive 1.5mm of additional locking compression, use the gold drill guide, specifically designed for this technology. If compression is not desired, the Presslock® drill-guide can be reversed and used in the ‘neutral’ position with the arrow facing away from the bone segment.

Monoaxial & Polyaxial System
The Airlock® Ø3mm and Ø3.5mm locking and non-locking screws may be used in all Airlock plate fixation threaded holes. However the standard compression hole only accomodate Ø3mm non-locking screws, and the Presslock® compression hole only accomodate Ø3.5mm locking screws.

- Monoaxial locking screws
- Polyaxial non-locking screws
- Tapered head
- Self-tapping design
- Self-retaining driver / screw interface
Compression Screws
Additional compression can be achieved with a combined Nexis Ø4mm headless compression screw, beveled PECA Compressive Ø4mm screw, or Nexis Ø5mm headless compression screw.
All instrumentation is conveniently organized and color coded.

Airlock with Presslock Upgrade
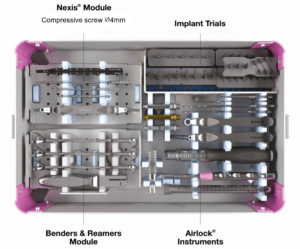
Airlock 2.0
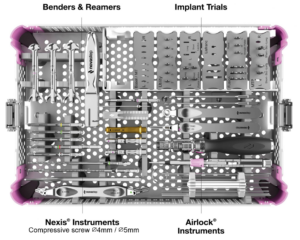
Airlock 2.0 Reduction Instruments
Prior to use Novastep® systems, carefully read the surgical technique, the instructions for use (IFU) and all packaging label information related to the implants and instruments.
Medical devices. Implants: Class IIb-CE1639; Instruments: Class I-CE / Class Ir-CE1639 / Class IIa-CE1639.