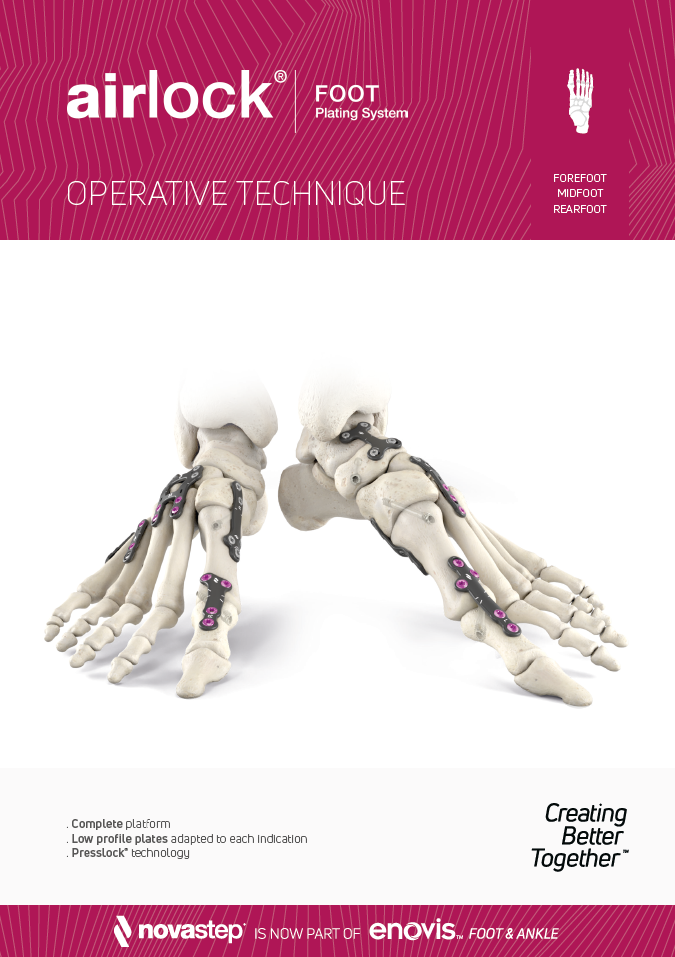
Utility
The versatile, low-profile utility plates are 1.5mm thick, allowing for usage in nearly all soft tissue environments and are available in a range of sizes.
The oblong standard compression hole accommodates a Ø3mm non-locking screw. Locking and non-locking screws of Ø3mm and Ø3.5mm may be used in all other fixation threaded holes
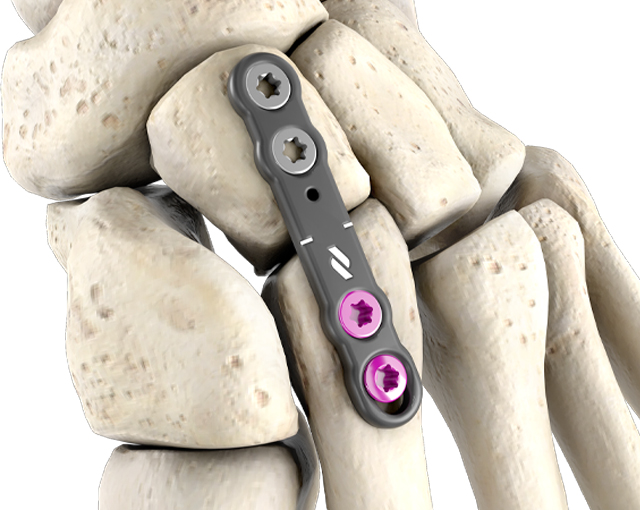
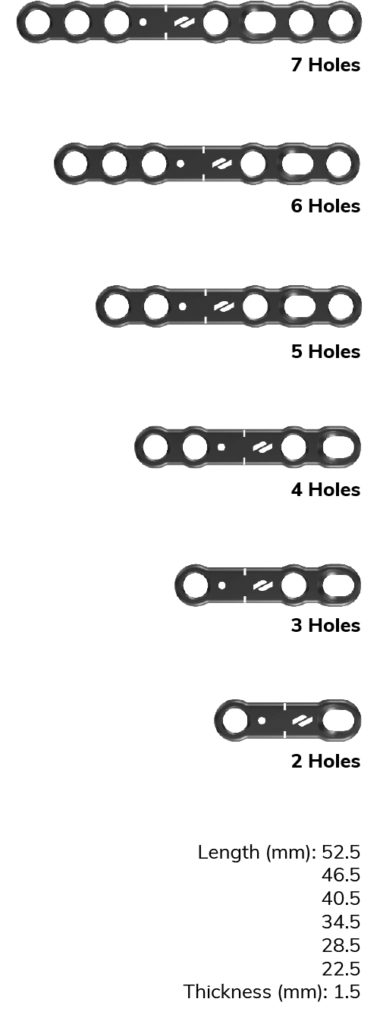
Utility
The versatile, high-strength, low-profile utility plates are 1.5mm thick, allowing for usage in nearly all soft tissue environments and are available in a range of sizes.
The oblong standard compression hole accommodates a Ø3mm non-locking screw. Locking and non-locking screws of Ø3mm and Ø3.5mm may be used in all other fixation threaded holes
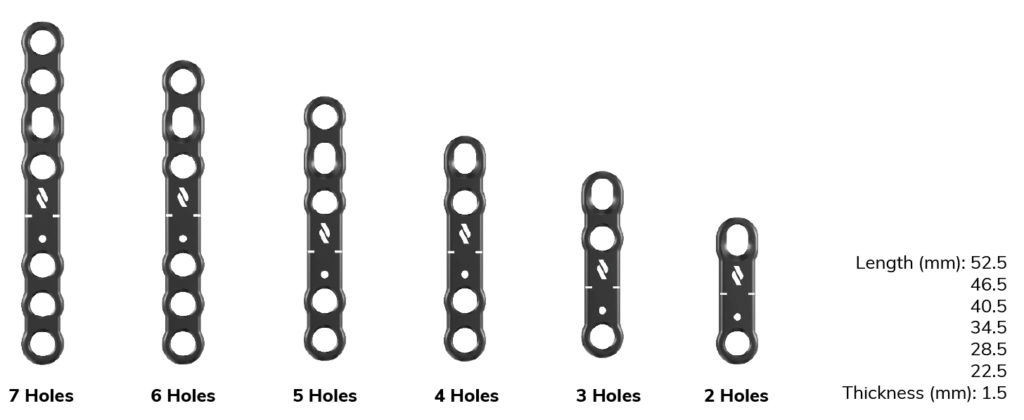

Low profile design > Plate thickness optimized according to the indications to limit subcutaneous discomfort.

Monoaxial & Polyaxial System
The Airlock® Ø3mm and Ø3.5mm locking and non-locking screws may be used in all Airlock plate fixation threaded holes. However the standard compression hole only accomodate Ø3mm non-locking screws, and the Presslock® compression hole only accomodate Ø3.5mm locking screws.

- Monoaxial locking screws
- Polyaxial non-locking screws
- Tapered head
- Self-tapping design
- Self-retaining driver / screw interface
Compression Screws
Additional compression can be achieved with a combined Nexis Ø4mm headless compression screw, beveled PECA Compressive Ø4mm screw, or Nexis Ø5mm headless compression screw.
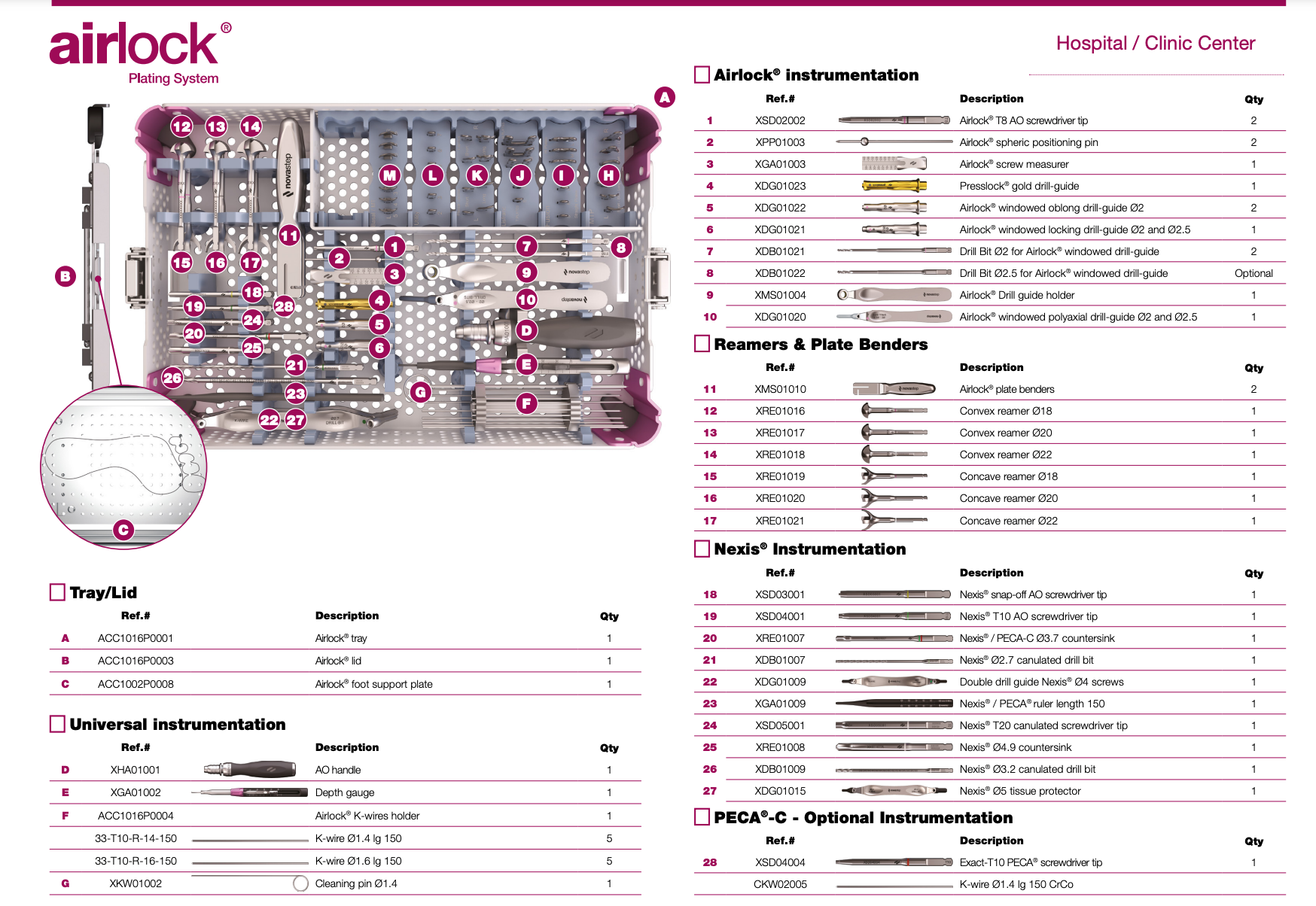
All instrumentation is conveniently organized and color coded.

Airlock with Presslock Upgrade
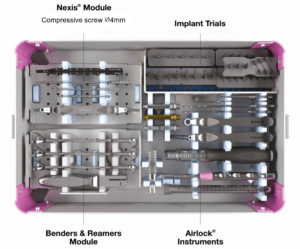
Airlock 2.0
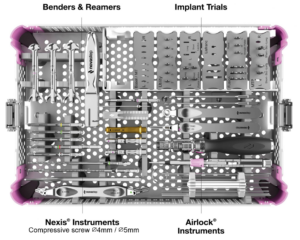
Airlock 2.0 Reduction Instruments
Related Products
Prior to use Novastep® systems, carefully read the surgical technique, the instructions for use (IFU) and all packaging label information related to the implants and instruments.
Medical devices. Implants: Class IIb-CE1639; Instruments: Class I-CE / Class Ir-CE1639 / Class IIa-CE1639.